15 May, 2018 Lecture by A. Polyansky on "Atomistic mechanism of the constitutive activation of PDGFRA via its transmembrane domain" and short students' talks.
On Tuesday, May 15, seminar "Supercomputer and Multiscale Modelling of Condensed Phase and Biological Systems" was held. A. Polyansky from IBCh RAS gave a talk "Atomistic mechanism of the constitutive activation of PDGFRA via its transmembrane domain". The result of this study was the molecular model of PDGFRa receptor activation, and the role of its oncogenic mutant form have been revealed. On the second part of the seminar students of SAMMA Laboratory reported on their projects.
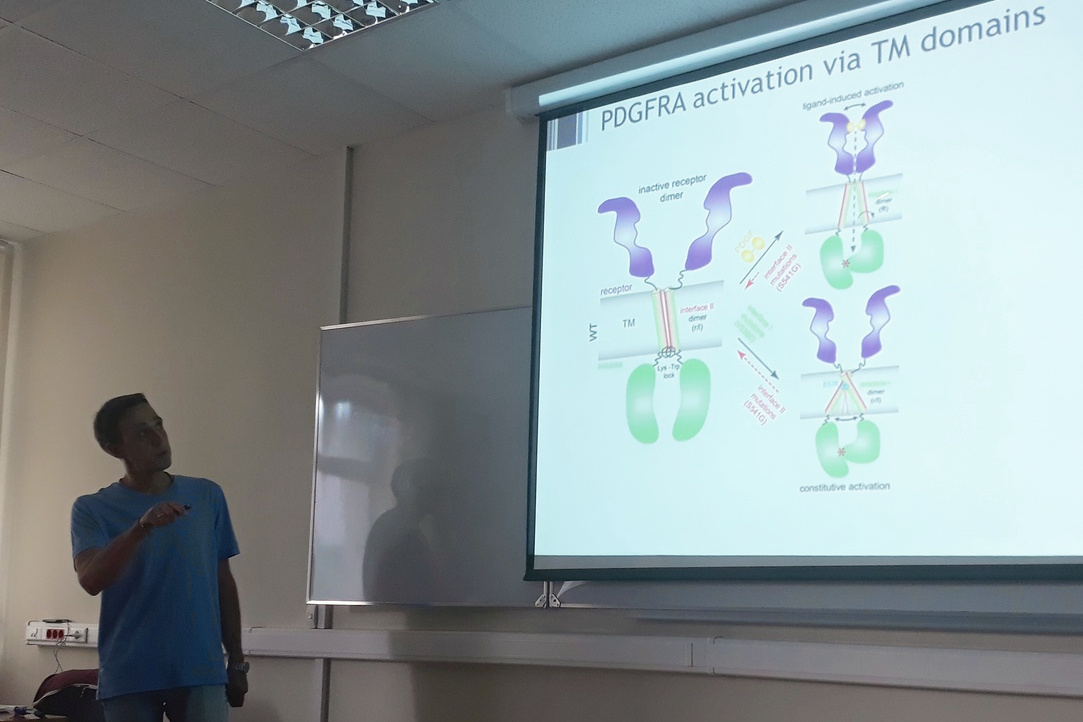
Receptor tyrosine kinases (RTKs) are an important class of single-pass membrane proteins, which control cell proliferation and growth. Upon dimerization, the transmembrane domains of RTKs transmit an allosteric signal from extracellular receptor regions bound to specific ligands to intracellular kinase domains. Mutations in transmembrane domains may lead to constitutive activation of these receptors and cause a number of severe disorders including cancer. To probe the mechanistic aspects of activation of RTKs via mutations in their transmembrane domains, we focus on platelet-derived growth factor receptor alpha (PDGFRA), an RTK which plays essential roles in embryo development and is aberrantly activated in a number of neoplasms. We use a computational modeling framework including sequence-based structure prediction and atomistic molecular dynamic simulations to identify and characterize novel mutations modulating dimerization of transmembrane domains and subsequent activation of the receptor, and validate our models by NMR spectroscopy and cell experiments. A synergy of modeling and experimental work allows us to elucidate the structural and dynamic determinants of spontaneous activation of PDGFRA and propose a model for the active and inactive conformational states of its TM dimer in both the wild type and the oncogenic mutant of the receptor.
Junior Research Fellow
Research Assistant
Research Assistant
Research Assistant
